Prostate Cancer Screening: What is the PCA3 Test?
-
Prostate cancer is the second leading cause of cancer death in men in the United States. The Federal Drug Administration (FDA) approved a type of blood test, prostate specific antigen (PSA), in 1986.
Since then, PSA has been widely used for prostate cancer screening and has led to the over diagnosis and treatment of non-lethal types of prostate cancer in a significant number of men. As a result, in 2012, the United States Preventive Services Task Force recommended against routine screening for prostate cancer in men. While this recommendation has been praised by some clinicians, urologists have generally criticized it. This has led to confusion among men as to the benefits of prostate cancer screening using the PSA test.
PSA was originally developed to monitor for prostate cancer reoccurrence after prostate cancer surgery (open or robotic prostatectomy) and not as a screening tool. Although elevated PSA can suggest presence of prostate cancer, PSA can also be elevated by other conditions such as enlarged prostate (known as BPH), difficulty urinating (urinary retention), and inflammation or infection of the prostate (prostatitis).
It is often difficult for a physician to determine the appropriate course of action for men who have had one or more prior negative prostate biopsies but continue to have a consistently elevated or rising PSA. While repeat biopsy of the prostate is often recommended, this procedure is not without risk. Although side effects of prostate biopsies are uncommon, they can include infection, blood in urine (hematuria), blood with ejaculation (hematospermia), rectal bleeding, and urinary retention.
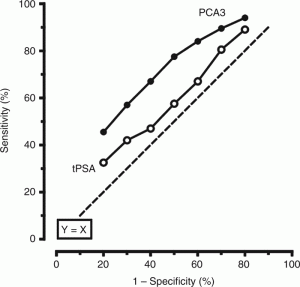
The PCA3 test may reduce the need for biopsies and improve patient health outcomes for prostate cancer.
Over the past decade, a number of new tests have been introduced that can improve the accuracy of prostate cancer detection and differentiation of non-aggressive from aggressive types of cancer. One of the most common and non-invasive tests is the urinary Prostate Cancer Antigen 3 gene (PCA3). After the doctor performs a digital rectal exam of the prostate in the office, the first urine sample (20-30 ml) is collected and then is shipped to the designated testing sites. This urine test provides a value (ranging from 0-100) that a urologist can use to determine if a repeat prostate biopsy is advisable.
PCA3 should not replace PSA blood test for prostate cancer screening, but it can serve as a useful supplement to PSA, digital rectal exam, and clinical judgment of the urologist. These tools can prevent unnecessary biopsies and treatment and help identify patients who need treatment the most.
Learn about the Risk Assessment Program at Fox Chase Cancer Center as well as prostate cancer diagnosis and treatment at Fox Chase Cancer Center.
For more information on PCA3 and its role in prostate cancer screening please click on the link below to access Dr. Reza Mehrazin and Dr. Marc C. Smaldone’ s recent article in Renal and Urology News.
